When ocular allergy occurs with low-dose atropine use, how should it be managed? Curious as to whether other clinicians have experienced a similar case, PC posted a photo of a child with red eyes after 2 weeks use of low-dose atropine on our Facebook discussion group.
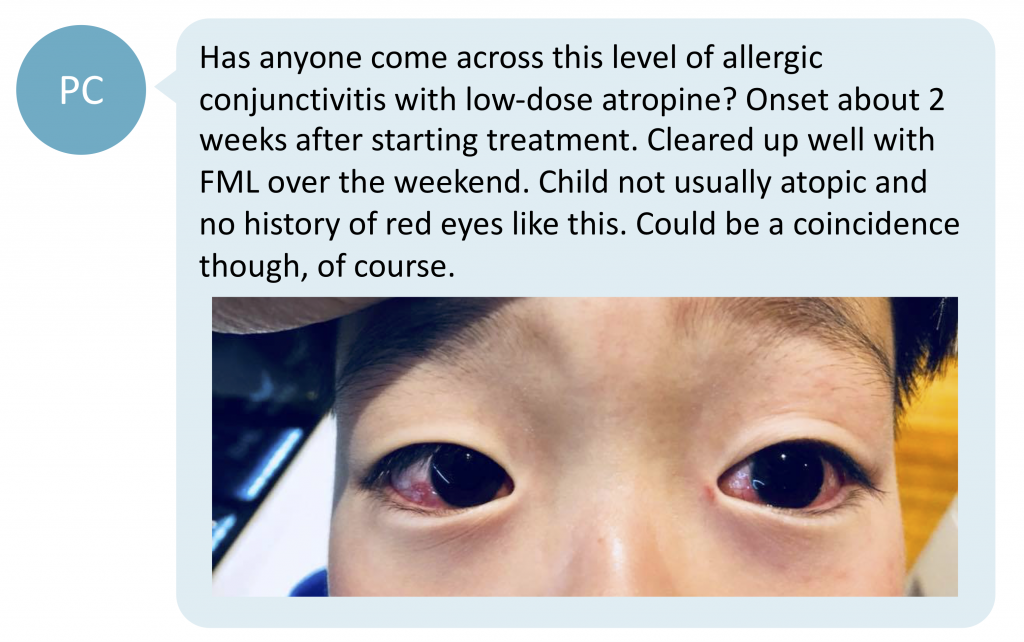
What are the considerations?
Patient history and use of other medications

The key message here is to check for history of recent health issues or other eyedrops used to rule out other possible causes of the red eyes.
Potential causes of allergic reaction

- Allergic reaction to preservative
Many suggested the allergic reaction is due to preservative toxicity. The type of preservative and its concentration can affect the extent of allergic reaction. Some suggested to contact the compounding chemist to confirm the concentration of atropine and any preservatives used in the drops that were dispensed to the patient. Benzalkonium chloride (BAK), thimerosal and chlorobutanol are the common preservatives1 in multidose topical eye drops which can be associated with ocular hypersensitive reaction manifested in the forms of ocular dryness, ocular surface damage, blepharitis and contact dermatitis.2 The next logical step is then to ensure that non-preservative atropine is dispensed to the patient. - Allergic reaction to atropine
Whilst uncommon, this is still possible. Kothari et al3 describes the common signs and symptoms of allergy to atropine to be itching, burning, periocular redness and eyelid swelling. However, these reactions were not limited to those on preservative-free formulations. They found that the severity of this response was correlated with concentration of atropine echoing the findings in the ATOM2 study4 whereby allergic conjunctivitis and dermatitis was noted in the 0.1% and 0.5% group, and none in the 0.01% group. The LAMP study reported 'occurrence of allergic conjunctivitis being similar among all groups' indicating no difference in rates between the placebo, 0.01%, 0.025% or 0.05% formulations.5
Communicating with compounding pharmacies

Some have suggested calling compounding pharmacies to understand their formulation of these drops. The aqueous base, amount and type of preservatives (if any) may vary between pharmacies, so it is useful to understand which other non-active ingredients are going into the drops that are ultimately dispensed to the patient.
Take home messages
- Consider other differential diagnoses - in this case viral conjunctivitis or another medication toxicity was suggested.
- It is more likely for a patient to be allergic to preservatives than atropine. As atropine is prescribed in such low concentrations for myopia control, this makes the case for a reaction to atropine quite unlikely.
- Ensure that the patient is dispensed a preservative-free formulation to avoid potential long-term effects of preservative toxicity.
- Get familiar with the ingredients (and any preservatives) used in formulations at your local compounding pharmacies.
- Communicate the symptoms to watch for with potential allergic reactions to the patient and parents, to avoid any nasty surprises down the road.

About Connie
Connie Gan is a clinical optometrist from Kedah, Malaysia, who provides comprehensive vision care for children and runs the myopia management service in her clinical practice.

About Kimberley
Kimberley Ngu is a clinical optometrist from Perth, Australia, with experience in patient education programs, having practiced in both Australia and Singapore.
Read our six-part blog series on atropine
Check out this additional clinical case
You can also listen to our three podcasts on atropine with world-leading researchers
-
Atropine, engaging with science and responsible practice with Professor Karla Zadnik from Ohio State University, USA. (link)
-
More on atropine 0.01% treatment for myopia management with Professor Mark Bullimore from the University of Houston, Texas USA. (link)
-
Atropine 0.01% for myopia management with Professor James Loughman from Technological University Dublin, and the Centre for Eye Research Ireland. (link)
This content is brought to you thanks to an unrestricted educational grant from

References
- Hong J, Bielory L. Allergy to ophthalmic preservatives. Current opinion in allergy and clinical immunology. 2009 Oct 1;9(5):447-53. (link)
- Coroi MC, Bungau S, Tit M. Preservatives from the eye drops and the ocular surface. Rom J Ophthalmol. 2015 Jan-Mar; 59(1): 2–5. (link)
- Kothari M, Jain R, Khadse N, Rathod V, Mutha S. Allergic reactions to atropine eye drops for retardation of progressive myopia in children. Indian J Ophthalmol. 2018 Oct; 66(10): 1446–1450. (link)
- Chia A, Chua WH, Cheung YB, Wong WL, Lingham A, Fong A, Tan D. Atropine for the Treatment of Childhood Myopia: Safety and Efficacy of 0.5%, 0.1%, and 0.01% Doses (Atropine for the Treatment of Myopia 2). Opthahlmology. 2012 Feb;119(2):347-54 (link)
- Lam JC, Jiang Y, Tang SM et al. Low-Concentration Atropine for Myopia Progression (LAMP) Study. Ophthalmology 2019;126:113-24. (link)















